Which Equilibrium System Least Favors The Formation Of Products?
Which equilibrium system least favors the formation of products?. Le Chateliers Principle. Which equilibrium system least favors the formation of products. Correct answer to the question Which equilibrium system least favors the formation of products.
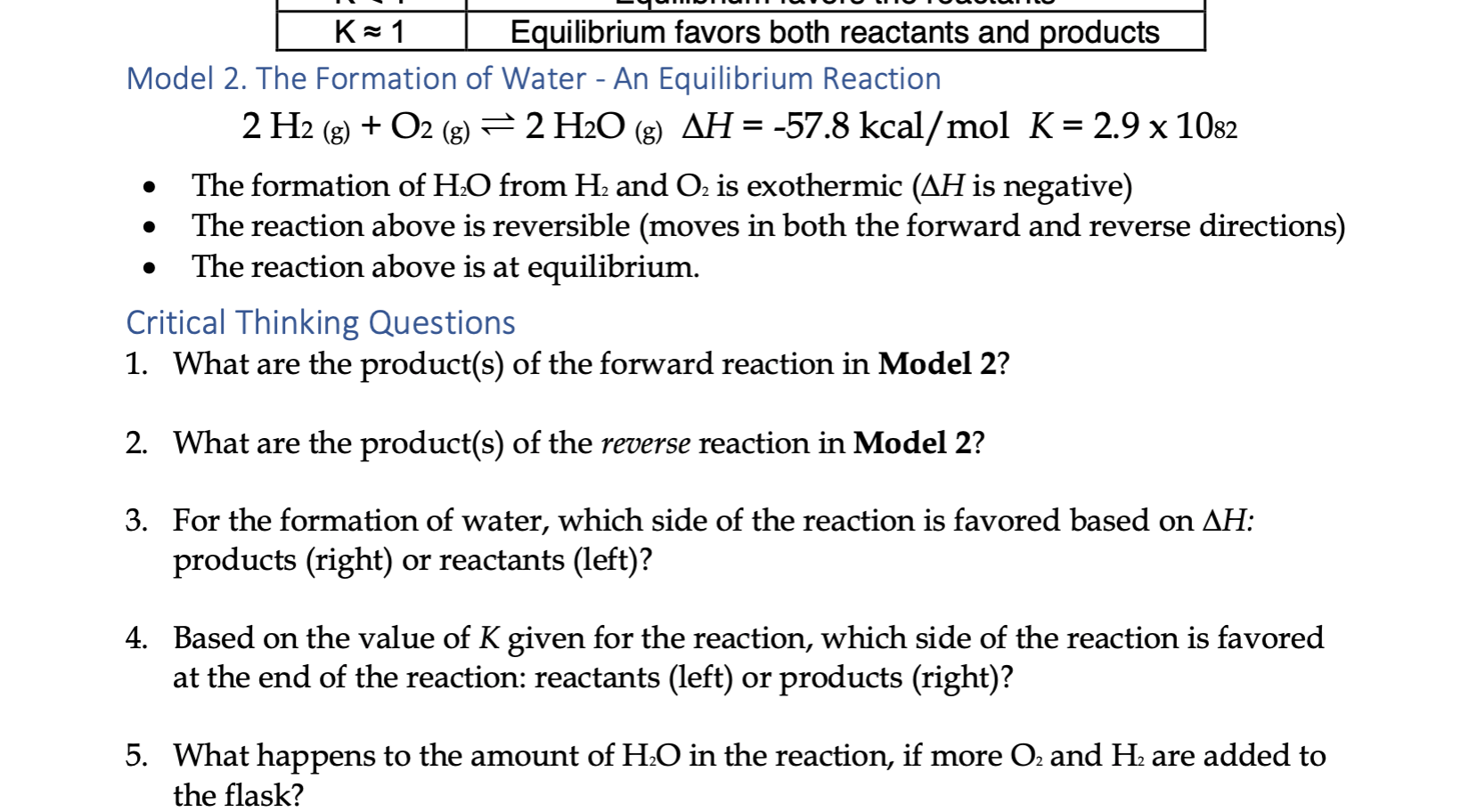
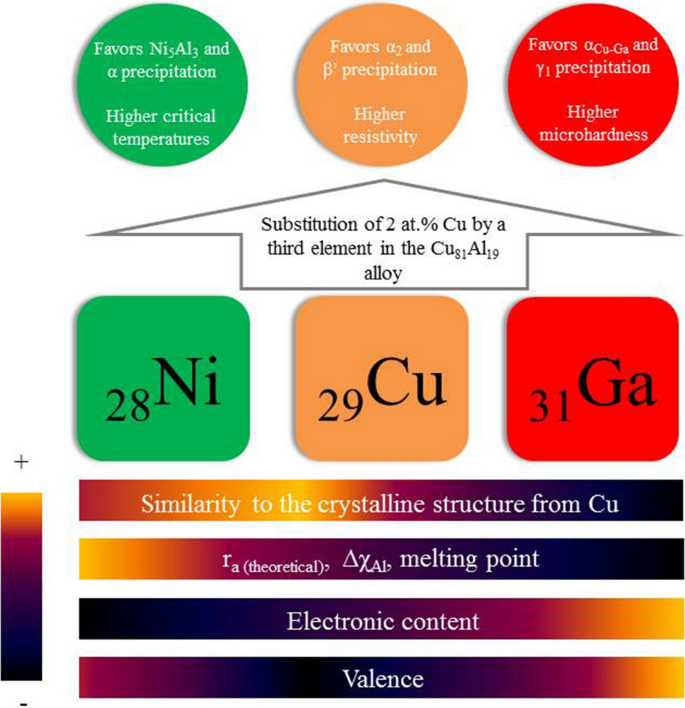
For a given stoichiometric equation which equilibrium constant represents a reaction that favors the formation of the products to the greatest extent. Predict at least two circumstances outside influences that would cause a system in equilibrium to change the concentrations of its reactants or products. The equilibrium system N_2O_4s hArr 2NO_2g has K_p11 For which equilibrium system is K_p0.
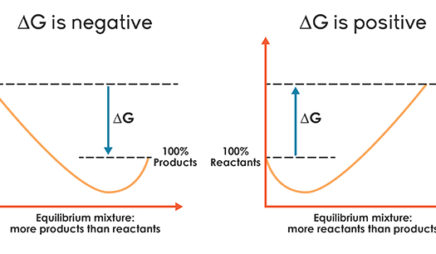
Chemical equilibrium was studied by the French chemist Henri Le Chatelier 1850 - 1936 and his description of how a system responds to a stress to equilibrium has become known as Le Chateliers principle. 1 question Which equilibrium system least favors the formation of products. If a reaction is not at equilibrium you can use the reaction quotient Q to see where the reaction is in the pathway.
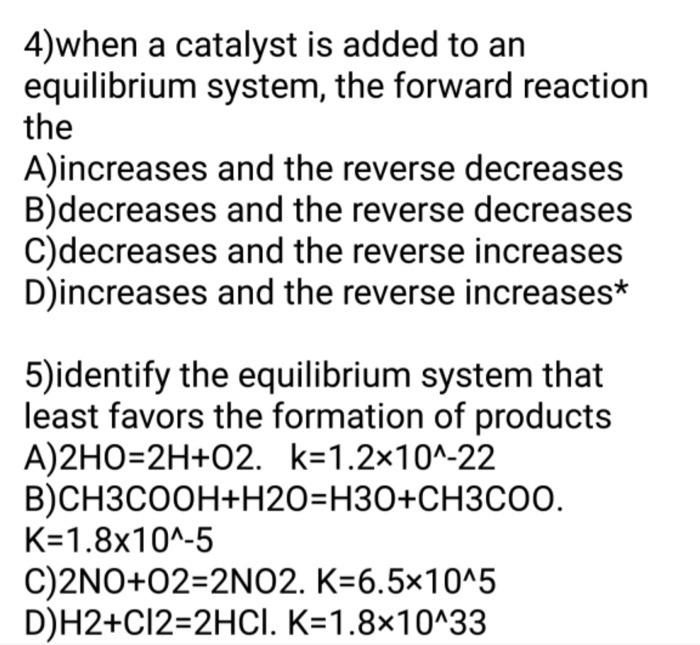
Which of the following values of heat of formation indicates that the product is least stable Which of the following values of enthalpy of formation indicates that the product is least stable. 2hgos 2hgl o2g keq 12 x 10-22 ch3coohaq h2ol h3oaq ch3coo- aq keq 18 x 10-5. When a chemical system that is at equilibrium is disturbed by a stress the system will respond in order to relieve the stress.
The system is then said to be in its equilibrium state or more simply at equilibrium. If Q K the reaction is at equilibrium. 2HgOs 2Hgl O2g Keq 12 x 10-22 CH3 Get the answers you need now.
O Keg 100 o Keg 10 x108 O Keg 10 x 10-3 O Keg 10 x10-18. An equilibrium constant greater than 1 for a reaction indicates that the reaction favors formation of the products Kc5 x10-¹⁰ Which of the following equilibrium constants indicates the reaction that gives the smallest amount of product. If Q K the reactants are favored.
A Keq 10 10-3 B Keq 10 108 C Keq 10 10-18 D Keq 100 B. A chemical reaction is in equilibrium when there is no tendency for the quantities of reactants and products to change.
If a reaction is not at equilibrium you can use the reaction quotient Q to see where the reaction is in the pathway.
If Q K the reaction is at equilibrium. Its also important to realize that molecules never stop moving even when equilibrium is reached. Which of the following values of heat of formation indicates that the product is least stable Which of the following values of enthalpy of formation indicates that the product is least stable. 2HgOs 2Hgl O2g Keq 12 x 10-22 CH3 Get the answers you need now. If Q K the products are favored. Which equilibrium system least favors the formation of products. 1 question Which equilibrium system least favors the formation of products. Eventually this change will stop after which the reaction composition will remain unchanged as long as the system is not disturbed. A chemical reaction is in equilibrium when there is no tendency for the quantities of reactants and products to change.
Le Chateliers Principle. Which equilibrium constant represents a reaction that favors the formation of the products to the greatest extent. The system is then said to be in its equilibrium state or more simply at equilibrium. If Q K the products are favored. An equilibrium constant greater than 1 for a reaction indicates that the reaction favors formation of the products Kc5 x10-¹⁰ Which of the following equilibrium constants indicates the reaction that gives the smallest amount of product. Which of the following values of heat of formation indicates that the product is least stable Which of the following values of enthalpy of formation indicates that the product is least stable. Its also important to realize that molecules never stop moving even when equilibrium is reached.



















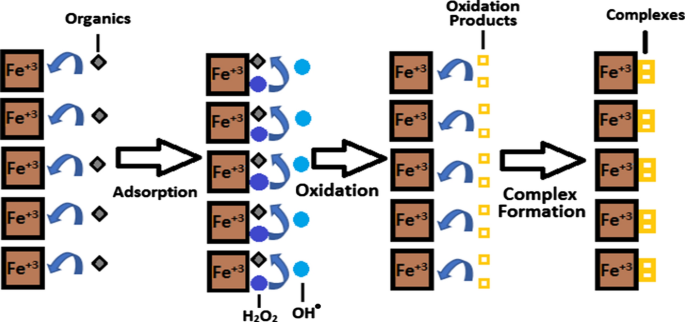
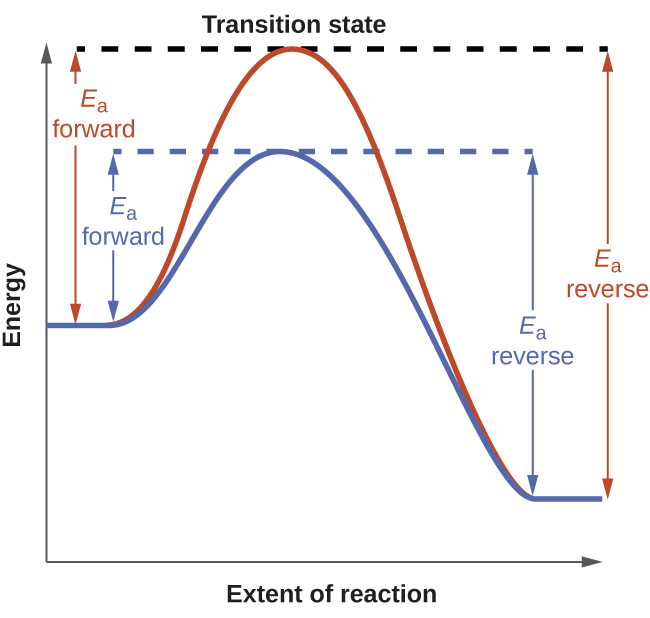


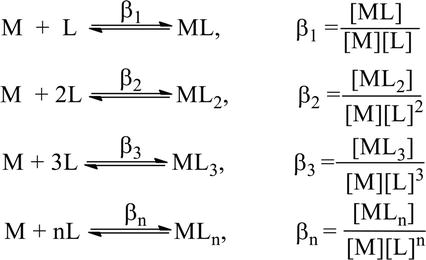



Post a Comment for "Which Equilibrium System Least Favors The Formation Of Products?"